About the Team
Industry: Medical Devices
Product: Devices for advanced biopsy diagnostics and cardiovascular intervention procedures using TransMed7’s Zero5® technology
Location: Portola Valley, CA
Why Fictiv
Customer Challenges: Needed faster and more cost-efficient turnaround on parts to iterate on prototype designs more quickly and a partner who could seamlessly scale production to meet customer demand.
Capabilities Leveraged: Selective Laser Sintering (SLS) 3D printing, CNC, Injection molding
Result: Cut design and development from weeks to days, and production from months to weeks. Accelerated final design commercial NPI from 10 to 2 years for five new breakthrough medical devices. Saved millions in infrastructure and overhead costs with Fictiv’s Digital Manufacturing Ecosystem.

Building Breakthrough Medical Technology
TransMed7 was founded to bring new diagnostic and therapeutic medical device solutions for diseases around the world — with a focus on oncology, regenerative medicine, and cardiovascular interventions. Their technology can accurately retrieve multiple pieces of tissue with a single insertion while being the least invasive, least disruptive way to get a piece of tissue out of a human body and to a lab for complete diagnosis and therapy planning.
Their mission is to get these breakthrough medical technologies in the hands of doctors around the world — and start saving lives as fast as possible.
“Patients have had to suffer with the same biopsy technology that’s been around for the past 40 years,” said Gene Vetter, TransMed7’s CEO. “Every day that passes means another patient is not getting the best information about their cancer diagnosis.”
Choosing A Faster, Leaner Path to Market
“We knew from inception that we intended to field multiple new next-generation technologies and that the traditional development cost to market numbers scenario would be prohibitive. Recent studies show that the average cost to bring a single new Class II medical device from initial concept to FDA 510(k) clearance before market launch is $31 Million USD,” said Vetter.
“We knew from inception that we intended to field multiple new next generation technologies, and that the traditional development cost to market numbers scenario would be prohibitive.”
– Gene Vetter, Co-Founder & CEO of TransMed7

“So, we decided to take a different approach leveraging Fictiv’s cloud manufacturing network to match with our extremely talented, but lean and mean internal team. It enabled us to save millions of dollars in infrastructure, people, and time costs when compared to traditional development and supply chain strategies.”
“Fictiv enabled us to save millions of dollars in infrastructure, people and time costs when compared to traditional development and supply chain strategies.”
– Gene Vetter, Co-Founder & CEO of TransMed7
Reducing Cycles From Months & Weeks to Weeks & Days
With Fictiv, the team realized they could automate workflow tasks, such as vendor sourcing & management, quoting, and quality control to dramatically accelerate cycle times. Immediately, timelines went from 2-3 weeks for Selective Laser Sintering down to just 3-5 days, and from months for injection molding down to just weeks — without sacrificing quality.

This was huge, according to Robert Sauchyn, TransMed7 Lead Design Engineer. “Working with Fictiv let us keep the momentum going on product iterations. Before, we’d have to put projects down for weeks and not think about them, then waste time re-engaging with a particular project once the parts arrived.”
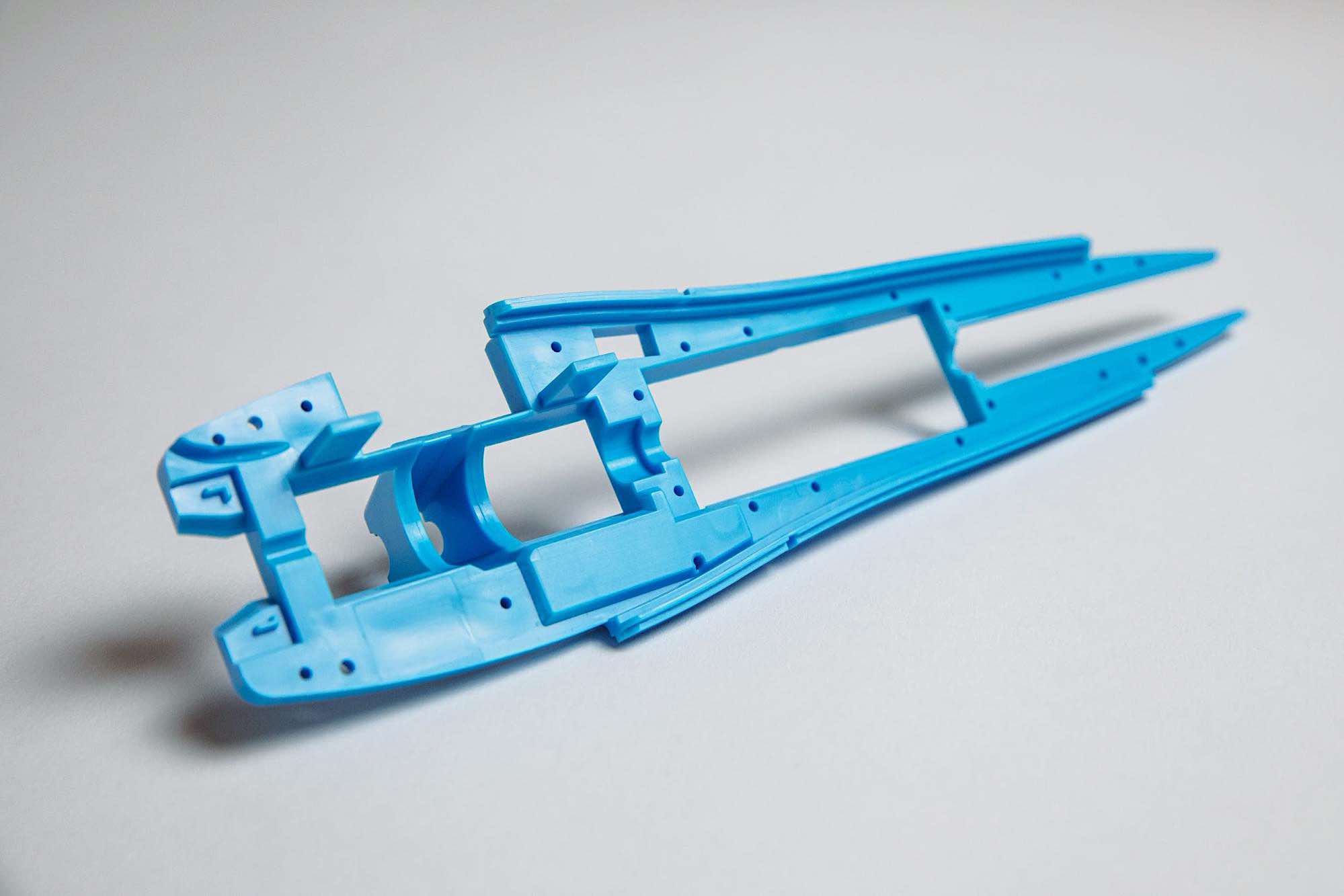
Exacting Quality Standards & Manufacturing Guidance
Because TransMed7 is commercializing medical devices, it has exacting quality, documentation, and inspection requirements. Every medical device must meet standards set forth by the FDA here in the US and by other regulatory bodies around the world.
“When you’re ready to scale, you have to have a very robust quality system,” according to Dr. James Vetter, TransMed7’s Co-Founder and Chief Medical Officer. As he worked with us, our quality system became another key selling point.
And in Europe especially, regulations for medical devices are focused on evaluating the manufacturing data of the devices. So, it was critical for TransMed7 to find a partner who not only has world-class quality assurance but has the capability to meet TransMed7’s quality expectations as well.
It wasn’t just the faster turnaround times that facilitated their product development. “It’s also about the communication,” according to Dr. Vetter. “They’re great about answering our questions and giving us immediate attention and feedback when we have issues. And everybody we speak with at Fictiv knows what they’re talking about.”
“They’re great about answering our questions and giving us immediate attendtion and feedback when we have issues.”
Dr. James Vetter, Co-Founder & Chief Medical Officer of TransMed7
“We meet every week with the folks at Fictiv, and they bring in the people that we need — whether they’re salespeople, technical people, materials people, or molding experts — as the process converts from low level, low volume, expensive parts to high volume parts that are consistent from part to part.”

That quick turnaround feedback has helped avoid issues, shorten the cycle times between design iterations, and smoothly transition from prototyping to production. “So we’ve actually been educated a fair amount by the people at Fictiv, and that’s helped us a lot,” said Dr. Vetter.
Staying Lean and Agile While Scaling
That level of engagement and communication has forged a relationship of trust and has truly unlocked TransMed7’s potential. Dr. Vetter has founded, scaled, and sold multiple medical device companies before TransMed7. Now that he’s seen the power of a digital manufacturing partner, he wouldn’t do it any other way.
“Typically, in the companies that I worked in previously, we did things differently. We had everything brought in house for new product development and that was normal back in the day because we wanted to control our own timelines.” But keeping everything in-house necessitates a lot of facilities, capital equipment, and overhead costs. “We were burning well over a million dollars a month even in the early stages of prototyping,” said Dr. Vetter.

With Fictiv’s help, TransMed7 has been able to save much of the time and money spent on building and maintaining that infrastructure, and instead focus their resources where it counts — developing their technology and getting it into the hands of doctors to help patients around the globe. And the economic impact of accelerating the time to market for these new products is clear.
“It took 10 years to bring new products to market at my previous companies,” reflects Dr. Vetter. “Fictiv has enabled us to shave 8 years off of that timeframe for our first two devices.”
“Fictiv enabled us to shave 8 years off of the product development timeframe for our first two devices”
– Dr. James Vetter, Co-Founder & Chief Medical Officer of TransMed7
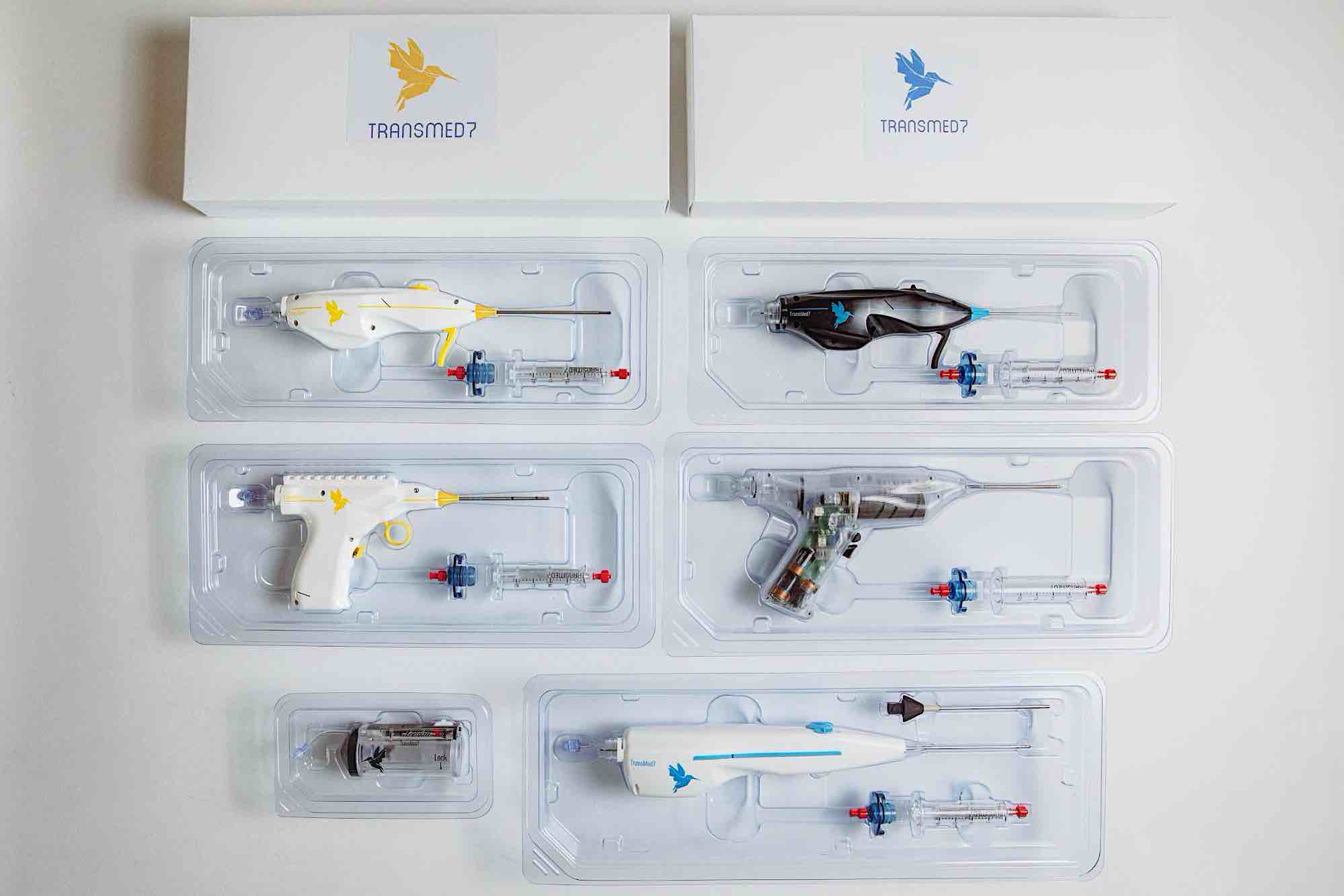
Sauchyn agrees.”Working with Fictiv has really allowed us to remain a lean team while simultaneously commercializing 5 breakthrough medical instruments, with 4 more on the way this year alone. It wouldn’t be possible without Fictiv’s help.”
Learn more about our enterprise solution for New Product Development Acceleration, then create your free account and try Fictiv for yourself!











