Article contents
▸
Article
Jorge Espinel
05.31.2021 Manufacturing Processes
Welcome to part II of our three-part series about secondary operations. This article covers finishing options, while the others discuss heat treatments and hardware installation.
When discussing CNC machining surface finishing options, you may have heard terms like as-machined, anodizing, powder coating, or media blasting. But before we dig into the details of those processes, we need to clearly define two terms that are time and again used incorrectly: surface finish and surface finishing.
Let’s clear up the confusion, shall we?
But first, a bit more on surface finish.
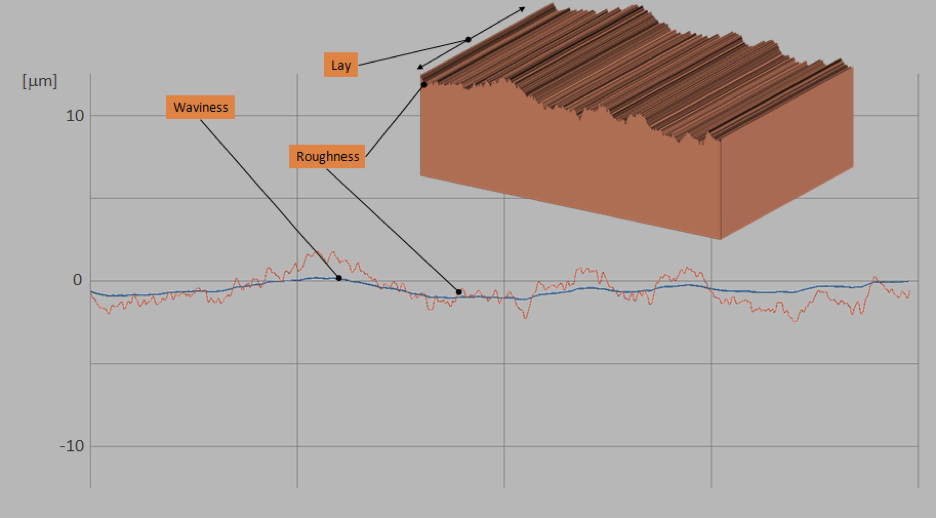
Surface finish should not be confused with the use of geometrical tolerances such as flatness, profile, or total runout. Different methods are used to measure surface finish, and the term describes the irregularities of a surface at the micro-level rather than dimensional inaccuracies. But you may wonder: if geometrical tolerances are defined on your drawing, and if the exterior surfaces of your machined component look ok, why bother with micro levels and layers?
CNC surface finish and finishing methods are particularly important if your part is in contact with other components. For example, the purpose of a ball-bearing is to reduce rotational friction and support radial and axial loads. When one of the races rotates, the balls also rotate because of the contact between them. If the surfaces of the balls or the races have the wrong surface finish characteristics, then friction increases, creating additional wear and reducing component lifespan – even when the components were fabricated within geometrical tolerances.
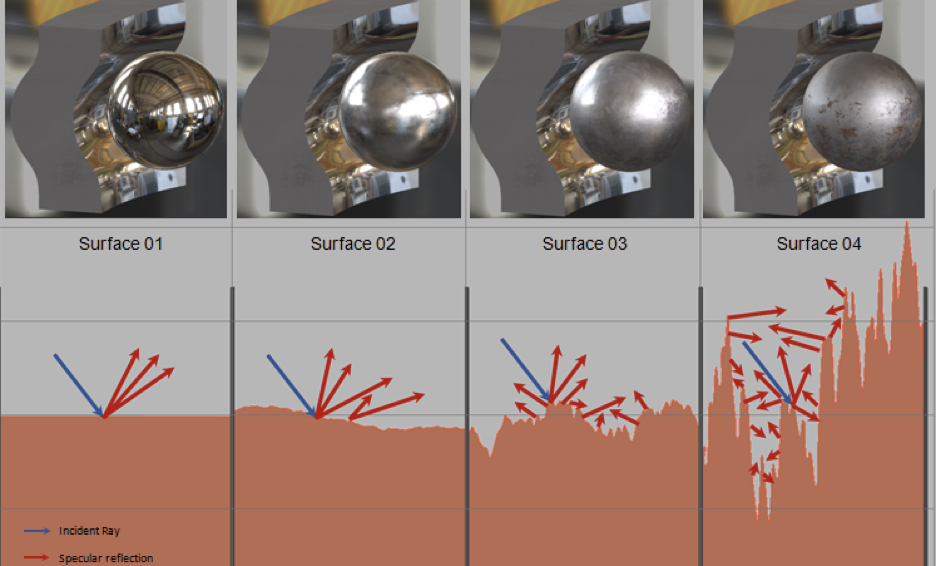
You can see a visual comparison of surfaces with different roughness values in the image below. In addition to changing the look, roughness plays a key role in contact mechanics since higher roughness values increase friction and cause faster wear on components. More roughness also means more surface irregularities that can become nucleation sites for corrosion and cracks. Still, higher values of roughness aren’t necessarily bad; when you’re interested in adhesion, roughness can be a benefit if you choose an appropriate material and surface finishing option.
Now that you understand surface finish characteristics, we should talk about the prep work that happens before surface finishing processes are applied.
Masking may be required to protect a surface or hole during the finishing process because some finishes add a layer of material, and that added thickness can interfere with tight tolerances, threaded holes, and press fits.
For holes less than one inch in diameter, the hole is plugged with a piece of rubber, which prevents the finish from interfering with a tightly toleranced diameter or threaded hole. This process is manual and time-consuming, so each hole that needs to be masked can add to the cost of the part. For holes that are larger than one inch, some manufacturers have large rubber plugs, but some will use surface masking liquid to paint the inside of the holes.
Speaking of surface masking liquid, it’s used to protect surfaces that require a different finish for aesthetic reasons, or because a surface mates with another part and must maintain a certain tolerance.
In order to mask a surface, a protective lacquer is painted onto the part, then cured by air for about a day. Then comes post-processing when finishes are applied, and another day is required for them to cure. Finally, the masking liquid is removed, leaving the unfinished surface bare. Surface masking invariably requires longer lead times because it’s a manual process that requires curing time.
So, you understand surface finish characteristics and pre-finishing prep work, now it’s time to talk about the many finishing options for CNC parts – from conversion coatings and plating to abrasive and polishing processes.
Passivation prevents corrosion in steel and stainless steel. The process involves a chemical treatment that removes free iron from the surface of the material, resulting in a smooth, shiny finish. It’s not a coating, so it doesn’t add any thickness to the part and therefore does not require masking.
Alodine™, the brand name of chromate conversion coating, is also known as chem film. It’s a thin coating used to passivate aluminum. The bath of chemicals used to apply the coating is often made with proprietary formulas, but all use Chromium as the main component. When requesting Chem film or Alodine™ for your machined parts, you might see the process is to MIL-DTL-5541F spec, which refers to the US Military Specification of Chemical Conversion Coatings on Aluminum and Aluminum Alloys.
Chem film or Alodine™ protective layer serves as a corrosion inhibitor and improves adherence for paints and adhesives, so it can be used in conjunction with decorative finishes. Chem film or Alodine™ also allows aluminum to maintain its thermal and electrical conductivity, while other finishes mitigate that conductivity. The color can be clear, gold, yellow, or tan, depending on the exact product used. It’s typically a cheaper process, and the coating is prone to scratches and superficial damage.
Similar to Chem film or Alodine™, anodizing is a passivation process that creates a protective layer on aluminum parts. The protective layer is formed using an acid electrolyte bath with a cathode passing electric current to the part, which serves as an anode (hence the name). Anodizing is a controlled way to oxidize a base material to improve durability and corrosion resistance. This outer layer is fully integrated with the substrate, so it does not flake or chip like paint and plating can. Due to the coating’s porous nature, anodized parts can also be dyed, painted, and sealed.
There are different types of anodizing: Type I, Type II, and Type III. Each is applied through a different process and results in different coating thicknesses and properties. All anodizing causes aluminum to be electrically non-conductive and prevents corrosion.
Black oxide is used on ferrous materials such as steel and stainless steel and it creates a layer called magnetite (Fe3O4) that provides mild corrosion resistance. It’s applied using a high-temperature chemical bath that contains alkaline cleaner, water, caustic soda, and a sealant such as oil to provide a smooth, matte finish. There are also variations of this process that function at cooler temperatures, but they offer less abrasion resistance. Masking is not necessary with black oxide as its application does not significantly affect the dimensions of the part.
This process is the deposit of a nickel-alloy coating by chemical reduction without using an electric current. Typical coatings are nickel-phosphorus where the higher phosphorus content improves corrosion resistance but decreases hardness. And if you’re using electroless nickel plating, do so only after heat treating to preserve the corrosion-resistant properties. Aluminum, steel, and stainless steel can all be electroless nickel plated.
There are two similar, but distinct types of zinc plating, and both are called galvanization. In general, both methods are used to protect steel from corrosion. When the coating is damaged and the material is exposed to the atmosphere, the underlying steel does not corrode because zinc is more reactive and oxidizes first.
Powder coating is used on steel, stainless steel, and aluminum, and is similar to painting your part. In this process, powdered paint is applied electrostatically to a part and either cured in an oven heated to 325-450 degrees or cured using ultraviolet light. Powder coating comes in a variety of colors and gloss levels and creates a thick, smooth uniform coating to provide increased durability.
Powder coating does change part dimensions, however, so tolerance and roughness value control are critical, and holes and mating surfaces with tight tolerances must be masked beforehand. Additionally, powder coatings have low electrical conductivity.
Electropolishing can be applied to steel or stainless steel and is how you can achieve a super fine or mirror finish on those materials. It uses an electric current and chemical bath to dissolve a tightly controlled layer of the base material. The electropolishing process involves myriad parameters, including base material chemical composition, electrolyte chemical composition, electrolyte temperature, time of exposure, and current density. You can fine-tune those parameters to achieve different levels of polish. The process is cheaper and faster than manual polishing.
Media blasting is an abrasive finishing process that removes debris and changes the surface roughness of parts. It works using a pressurized jet to fire an abrasive, usually glass or plastic beads or sand, toward the surface of a part. The effect is similar to using sandpaper, but it’s a faster process that provides a more even matte finish. It also works well finishing corners and fillets. A variation of this process uses water to lubricate the surface and trap dust, but bear in mind that using wet blasting on mild steel will result in immediate corrosion.
Media blasting can be used on most metals, including brass, bronze, and copper, and is often combined with other finishes, like anodizing, for its aesthetic benefits.
Tumbling, also known as barrel finishing, gives parts a matte finish by rotating them in a barrel filled with an abrasive or non-abrasive medium. It’s similar to media blasting but is less controlled, due to the larger size of abrasive pieces used. This makes it a lower fidelity process, generally used to remove burrs and sharp edges, and doesn’t work for parts smaller than 1 cubic inch (the size of the tumbling media).
It can be used on any metal and is a relatively cheap process. However, tumbling can create uneven sides and faces on parts, so be sure to check your geometrical tolerances requirements before selecting this option. For 3D printed applications, this process can correct artifacts and visible defects.
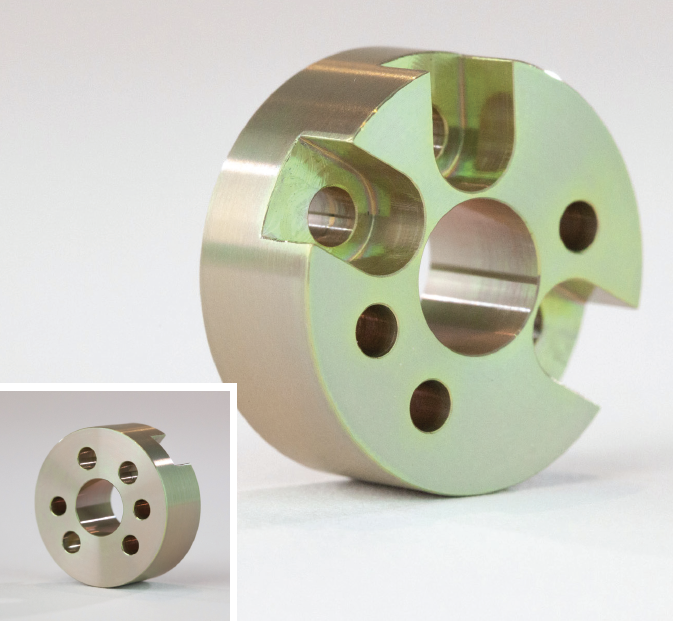
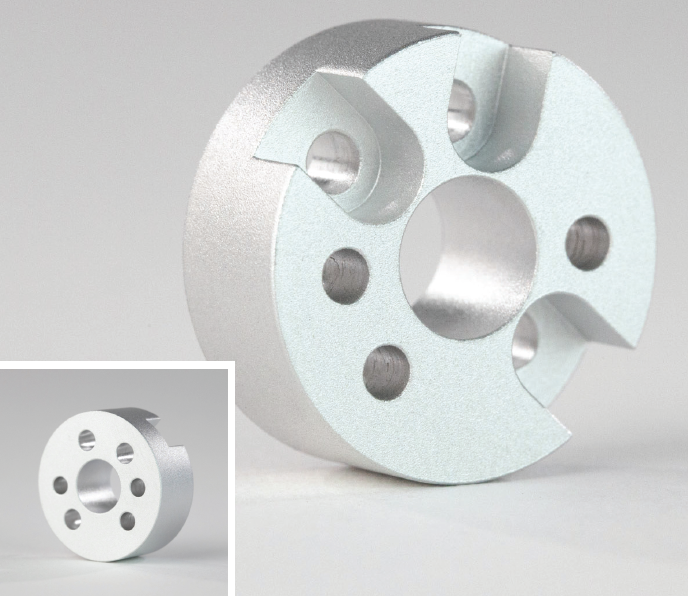
Multiple finishes can sometimes be combined to take advantage of their different properties. For example, media blasting is often done before applying other finishes to hide machining marks and give parts a smooth, matte finish. By combining media blasting with anodizing, for example, you’ll get the surface finish found on Apple’s MacBook laptops.
Type II anodizing and Chem film or Alodine™ can also be combined, though that combo requires masking areas you need to retain thermal and electrical conductivity. And passivation and black oxide are often applied in tandem to steel to provide both corrosion resistance and a nice cosmetic finish.
Hopefully, this article has helped you to understand the difference between surface finish and surface finishing, as well as introducing you to the various finishing options for CNC machined parts. To figure out which finishing options will deliver the right protection and aesthetics for your next project, remember to:
If you’re still having trouble figuring out which CNC machining surface finish is right for you, create a free account, and upload your design to get an online CNC quote and see what surface finishing options can be applied to your part. Our team of experienced technical application engineers can help, too!
By signing up, you agree to our Terms of Use and Privacy Policy. We may use the info you submit to contact you and use data from third parties to personalize your experience.